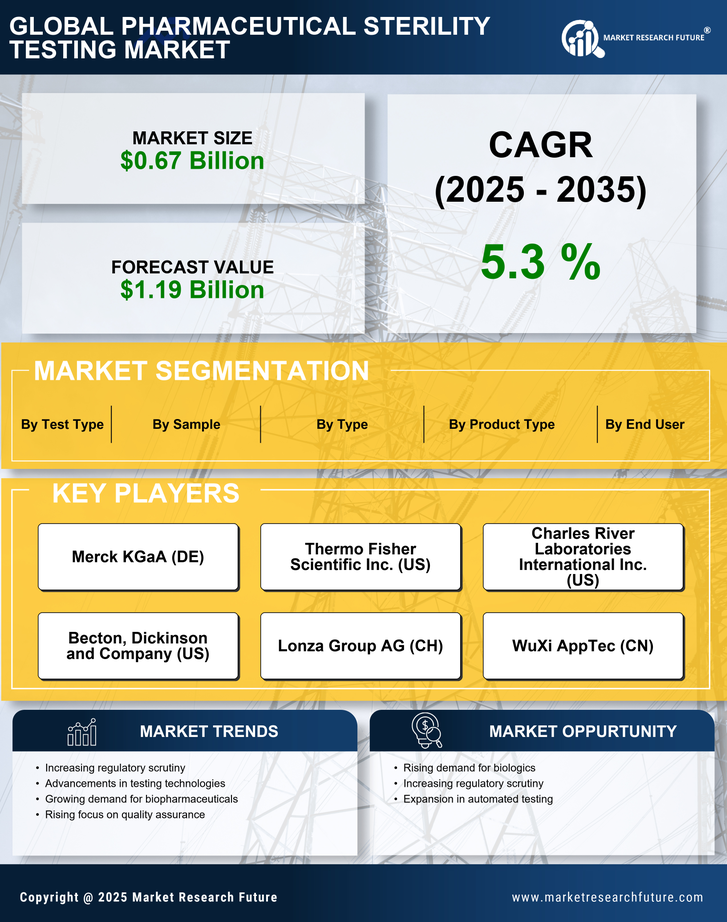
Regulatory Compliance and Quality Assurance Practices
Compliance requirements strongly influence growth within the Pharmaceutical Sterility Testing Market. Pharmaceutical manufacturers must demonstrate strict adherence to quality standards before sterile products can reach patients.
Sterility testing serves as a final checkpoint confirming that production environments remain contamination-free. Documentation accuracy is essential because regulatory inspections often review testing records in detail. Companies increasingly implement standardized operating procedures to maintain consistency.
Training programs play a major role in maintaining compliance. Personnel working in aseptic environments must follow strict gowning protocols and contamination prevention practices. Human error remains one of the most common contamination risks, making education essential.
Ref - https://www.marketresearchfuture.com/reports/pharmaceutical-sterility-testing-market-10720
Compliance requirements strongly influence growth within the Pharmaceutical Sterility Testing Market. Pharmaceutical manufacturers must demonstrate strict adherence to quality standards before sterile products can reach patients.
Sterility testing serves as a final checkpoint confirming that production environments remain contamination-free. Documentation accuracy is essential because regulatory inspections often review testing records in detail. Companies increasingly implement standardized operating procedures to maintain consistency.
Training programs play a major role in maintaining compliance. Personnel working in aseptic environments must follow strict gowning protocols and contamination prevention practices. Human error remains one of the most common contamination risks, making education essential.
Ref - https://www.marketresearchfuture.com/reports/pharmaceutical-sterility-testing-market-10720
Regulatory Compliance and Quality Assurance Practices
Compliance requirements strongly influence growth within the Pharmaceutical Sterility Testing Market. Pharmaceutical manufacturers must demonstrate strict adherence to quality standards before sterile products can reach patients.
Sterility testing serves as a final checkpoint confirming that production environments remain contamination-free. Documentation accuracy is essential because regulatory inspections often review testing records in detail. Companies increasingly implement standardized operating procedures to maintain consistency.
Training programs play a major role in maintaining compliance. Personnel working in aseptic environments must follow strict gowning protocols and contamination prevention practices. Human error remains one of the most common contamination risks, making education essential.
Ref - https://www.marketresearchfuture.com/reports/pharmaceutical-sterility-testing-market-10720
0 Yorumlar
·0 hisse senetleri
·2 Views